To calculate result you have to disable your ad blocker first.
Amount of Substance Calculator
Input the value of Mass (g) and Molar mass (g/mol) to find the amount of substance (Mass/Molar Mass) using this amount of substance calculator.
Amount of Substance Calculator
Amount of substance calculator is a tool designed to determine the quantity of a substance used to produce a product. It does this by taking into account the mass of the substance and its molar mass.
How to use this Amount of Substance Calculator?
To calculate the amount of substance in a mole follow the below steps:
- Input the value of Mass (g) and molar mass (g/mol).
- Press the calculate button.
- Click the show more button to see the step-by-step solution.
- Click the reset button to calculate another value.
This amount of substance calculator will provide you with the result in the number of moles that a substance has according to the input values i-e Mass and molar mass. Also, this calculator provides the solution with steps.
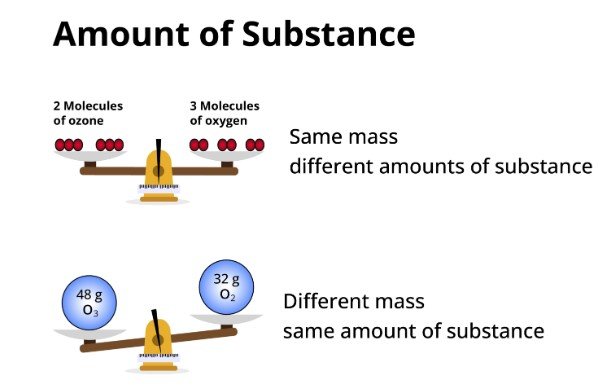
What is the amount of substance?
In the field of chemistry, there's a fundamental concept that aids in determining the amount of a substance needed for experiments in labs: the mole. The mole provides a measure of the quantity of a particular substance, expressed in terms of the number of particles present in a sample. This concept is pivotal for understanding the behavior of matter, whether on a microscopic or macroscopic scale.
The mole quantifies the 'amount of substance'. It is defined by Avogadro's number, which is 6.022×1023 particles, representing the number of constituent particles (usually atoms or molecules) that are contained in one mole of any substance.
Formula:
The mathematical formula of the amount of substance is stated as.
Amount of Substance = Mass (m)/Molar mass (M)
- Amount of Substance = m/Mm
- m = mass of the required substance
- Mm = Molar mass of the given substance
Examples
Here we solved some examples to explain the tool in detail.
Example 1:
Evaluate the required amount of substance of sulphuric acid (H2SO4) if its molar mass is approximately 36 and its mass is 72.
Solution:
Step 1:
Write the data from the question.
Molar mass = Mm = 36, Mass = m = 72, Amount of substance =?
Step 2:
Write the formula of the weight of the acid.
Amount of Substance = m/Mm
Where, m = mass of the required substance, Mm = Molar mass of the given substance
Step 3:
Putting all values in the above formula carefully and simplifying.
Mm = 36, m = 72,
Amount of Substance = m/Mm
= 72/36
Amount of Substance = 2 mole
Example 2:
Evaluate the required amount of substance of hydrochloric acid (HCI) if its molar mass is approximately 12 and its mass is 24.
Solution:
Step 1:
Write the data from the question.
Molar mass = Mm = 12, Mass = m = 24, Amount of substance =?
Step 2:
Write the formula of the weight of the acid.
Amount of Substance = m/Mm
Where, m = mass of the required substance, Mm = Molar mass of the given substance
Step 3:
Putting all values in the above formula carefully and simplifying.
Mm = 12, m = 24,
Amount of Substance = m/Mm
= 24/12
Amount of Substance = 2 mole
Example 3:
Evaluate the required amount of substance of hydrochloric acid (HCI) if its molar mass is approximately 34 and its mass is 24.
Solution:
Step 1:
Write the data from the question.
Molar mass = Mm = 34, Mass = m = 24, Amount of substance =?
Step 2:
Write the formula of the weight of the acid.
Amount of Substance = m/Mm
Where, m = mass of the required substance, Mm = Molar mass of the given substance
Step 3:
Putting all values in the above formula carefully and simplifying.
Mm = 34, m = 24,
Amount of Substance = m/Mm
= 24/34
Amount of Substance = 0.7059 mole

